Year after year, the number of patients with implanted medical devices (IMD’s), such as cardiac pacemakers or vascular stents, has been steadily increasing. MRI machines use powerful magnetic fields, and implants cannot be readily removed. Depending upon the interaction between the implanted devices and the magnetic fields, there is the possibility of injury and/or heat generation by way of the magnetic fields.
To prevent accidents, health care professionals must determine beforehand the MR safety information of implants to know whether or not a patient can safely receive an MRI examination.
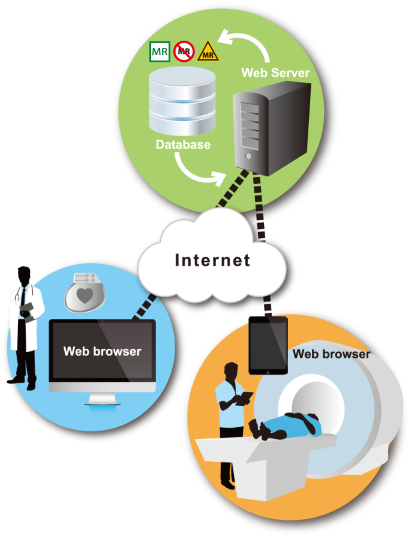
However, in Japan, health care professionals had the difficult task of making this determination without the benefit of an integrated database.
In March 2023, Medie released the new system named "Nextant🄬", which make it possible for health care professionals engaged in MRI examination to search for MR safety information on medical devices by product information. 'Nextant🄬' also enable them to search for the information by model/catalog/primary DI number, product name, device implantation site and/or surgical procedure name.
With regard to the MR safety information of IMD’s, Medie chose and applied one of the above five MR labeling, (which conforms to the language and terminology of the ASTM International "Standard Practice" F2503-20 and Establishing Safety and Compatibility of Passive Implants in the Magnetic Resonance (MR) Environment. Guidance for Industry and Food and Drug Administration Staff. Document issued on December 11,2014.) for each IMD, based on medical packaging inserts.
Further, Medie has placed information regarding MR labeling of MR conditional IMD’s into standardized categories. This allows health care professionals to easily verify information on MR safety.
The database is updated daily to provide the latest information.